Scientists from Jülich, the USA and the Czech Republic have developed an experimental set-up enabling electrochemical processes at solid-liquid interfaces to be monitored “live” and with unprecedented precision. As a result, numerous research problems can now be investigated with much higher accuracy than before, such as why lithium-ion batteries deteriorate or which catalyst materials would be most suitable for maximizing fuel conversion efficiency. The new platform resolves features as small as a few nanometres in size, has a temporal resolution of fractions of a second and is element- and oxidation state-specific; therefore it is able to distinguish between copper atoms and copper ions, for instance.
The experimental arrangement designed by scientists from Forschungszentrum Jülich, the National Institute of Standards and Technology (NIST), USA, Charles University in Prague as well as the University of Maryland and Oak Ridge National Laboratory (ORNL), both in the USA, is based on the well-established technique photoemission electron microscopy. Intrinsically, this powerful imaging technique is not suitable for studying liquids, as samples must be placed in a high vacuum.
“We devised a simple trick in order to protect the liquid in the samples from the vacuum”, explained Dr. Andrei Kolmakov from NIST. The solution, a so-called electrolyte, lies within thousands of tiny channels fabricated on a 0.5-millimeter-thick glass plate. The researchers then covered the plate with a layer of graphene, which acted like a transparent plastic wrap stretched over the openings of the channels.
Graphene not only prevents the liquid from evaporating, but the thin layer is also transparent for imaging with a photoemission electron microscope. In addition, graphene conducts electricity and acts as an electrode in the experiments. The scientists coated the bottom end of the channels with nanometre-thin films of platinum and closed them off with sealant, thus creating thousands of working electrochemical cells in miniature form. “The principle reported here can be adapted to fit very diverse studies on liquid-solid-gas interlayers”, explained Dr. Slavomír Nemšák from the Peter Grünberg Institute. The method is suitable for research questions in the areas of energy research, catalysis, magnetism and biomedicine, among others.
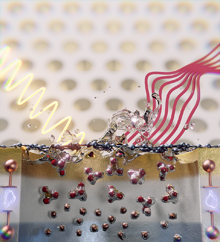
Top Image: Artistic representation of the experimental arrangement: in the foreground a section of a single electrochemical cell with the graphene-covered electrolyte in the middle, gold contacts at the top, and platinum contacts at the bottom. A photoemission electron microscope sends X-ray light (yellow wavy line) onto the sample and receives chemical information in the form of photoelectron spectra (red lines).
Copyright: Forschungszentrum Jülich